
What is Beer’s Law? This fundamental principle in chemistry delves into the relationship between the absorbance of light by a solution and the concentration of the substance within it. Beer’s Law, also known as the Beer-Lambert Law, is a cornerstone in analytical chemistry, providing a powerful tool for determining the concentration of a substance in a solution.
Imagine shining a beam of light through a colored solution. The amount of light that passes through depends on the color intensity, which is directly related to the concentration of the colored substance. Beer’s Law quantifies this relationship, stating that the absorbance of light is directly proportional to the concentration of the substance and the path length of the light beam through the solution. This simple yet profound law has numerous applications in various scientific fields, from determining the concentration of a drug in a sample to analyzing the purity of a chemical compound.
Introduction to Beer’s Law
Beer’s Law, also known as the Beer-Lambert Law, is a fundamental principle in spectrophotometry that establishes a relationship between the absorbance of light by a solution and the concentration of the analyte in the solution. This law is widely used in analytical chemistry for determining the concentration of substances in various samples, ranging from environmental monitoring to clinical diagnostics.
Beer’s Law states that the absorbance of a solution is directly proportional to the concentration of the analyte and the path length of the light beam through the solution. This means that as the concentration of the analyte increases, the absorbance of the solution also increases, and as the path length increases, the absorbance increases proportionally.
Key Variables in Beer’s Law
The relationship between absorbance, concentration, and path length is mathematically expressed as:
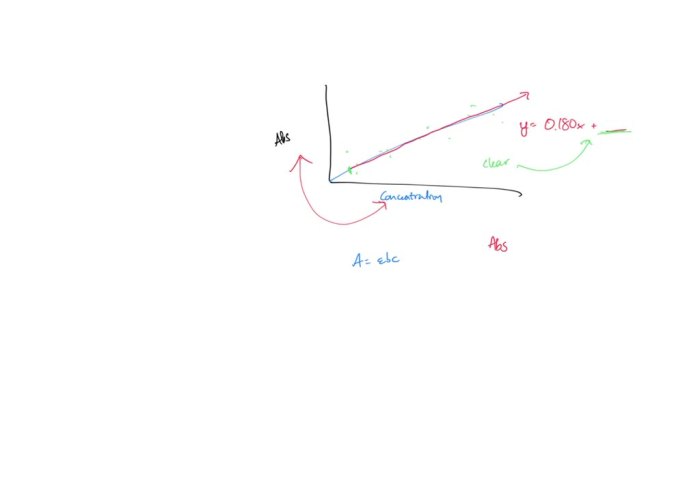
A = εbc
Where:
* A represents the absorbance of the solution, which is a dimensionless quantity measured by a spectrophotometer.
* ε represents the molar absorptivity, a constant specific to the analyte and the wavelength of light used. It is a measure of how strongly a substance absorbs light at a particular wavelength.
* b represents the path length, the distance the light beam travels through the solution. It is typically measured in centimeters (cm).
* c represents the concentration of the analyte in the solution, typically expressed in units of molarity (mol/L).
Understanding the Variables
- Concentration: The concentration of the analyte directly influences the absorbance. A higher concentration of the analyte leads to a greater number of analyte molecules interacting with the light beam, resulting in increased absorbance.
- Path Length: The path length of the light beam through the solution is also directly proportional to the absorbance. A longer path length means the light beam interacts with more analyte molecules, leading to a higher absorbance.
- Molar Absorptivity: Molar absorptivity is a constant that depends on the specific analyte and the wavelength of light used. It is a measure of how strongly the analyte absorbs light at a particular wavelength. A higher molar absorptivity indicates that the analyte absorbs light more strongly at that wavelength, leading to a higher absorbance for a given concentration and path length.
The Beer-Lambert Law and Its Applications
Beer’s Law is a fundamental principle in spectrophotometry that describes the relationship between the absorbance of a solution and the concentration of the analyte. However, the Beer-Lambert Law is a more comprehensive version of Beer’s Law, taking into account not only the concentration but also the path length of the light beam through the solution.
The Beer-Lambert Law
The Beer-Lambert Law states that the absorbance of a solution is directly proportional to the concentration of the analyte and the path length of the light beam through the solution. This relationship can be expressed mathematically as:
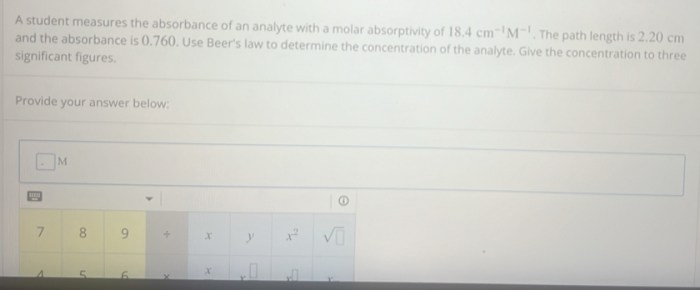
A = εbc
Where:
* A is the absorbance
* ε is the molar absorptivity, a constant that is specific to the analyte and the wavelength of light used
* b is the path length of the light beam through the solution
* c is the concentration of the analyte
Applications of the Beer-Lambert Law
The Beer-Lambert Law has numerous applications in various fields, including:
Chemistry
- Quantitative Analysis: The Beer-Lambert Law is widely used for quantitative analysis, such as determining the concentration of a substance in a solution. For example, in a titration experiment, the concentration of a titrant can be determined by measuring the absorbance of the solution at a specific wavelength.
- Purity Assessment: The Beer-Lambert Law can be used to assess the purity of a substance by comparing the absorbance of a sample to that of a known pure standard. Any deviation in absorbance can indicate the presence of impurities.
- Reaction Rate Studies: The Beer-Lambert Law can be used to monitor the progress of a chemical reaction by measuring the absorbance of the reactants or products over time.
Biology
- Protein Quantification: The Beer-Lambert Law is used to determine the concentration of proteins in solutions using techniques such as the Bradford assay and the Lowry assay.
- DNA and RNA Quantification: The Beer-Lambert Law can be used to quantify DNA and RNA samples using spectrophotometry at specific wavelengths.
- Enzyme Activity Assays: The Beer-Lambert Law is used to monitor the activity of enzymes by measuring the absorbance of the substrate or product of the enzymatic reaction.
Environmental Science
- Water Quality Monitoring: The Beer-Lambert Law is used to determine the concentration of pollutants in water samples, such as heavy metals, pesticides, and herbicides.
- Air Quality Monitoring: The Beer-Lambert Law can be used to monitor the concentration of gases in the atmosphere, such as ozone, sulfur dioxide, and nitrogen dioxide.
- Soil Analysis: The Beer-Lambert Law can be used to determine the concentration of nutrients and contaminants in soil samples.
Spectrophotometry and Beer’s Law: What Is Beer’s Law
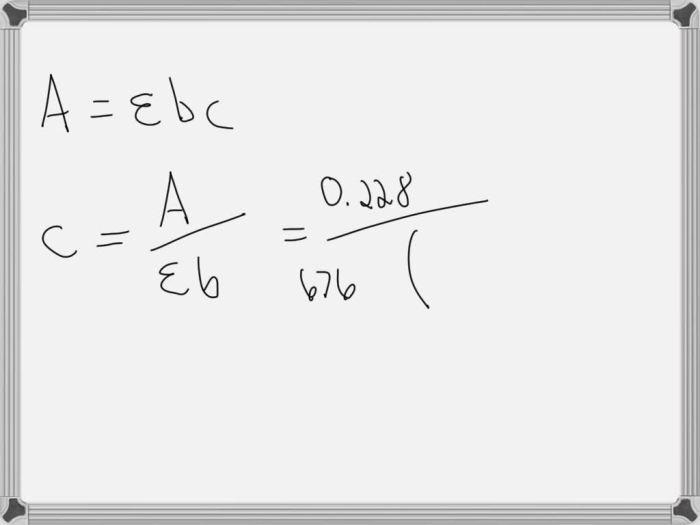
Spectrophotometry is an instrumental technique that plays a crucial role in applying Beer’s Law. It allows us to measure the absorbance of a solution at a specific wavelength, which is directly proportional to the concentration of the analyte according to Beer’s Law.
Principles of Spectrophotometric Measurements, What is beer’s law
Spectrophotometry is based on the interaction of light with matter. When a beam of light passes through a solution, some of the light is absorbed by the molecules in the solution, while the rest is transmitted through the solution. The amount of light absorbed is directly proportional to the concentration of the absorbing species.
The spectrophotometer works by shining a beam of light through a sample and measuring the amount of light that passes through it. The ratio of the incident light intensity to the transmitted light intensity is called the transmittance (T). Absorbance (A) is defined as the negative logarithm of the transmittance:
A = -log(T) = -log(It/Io)
where:
* Io is the intensity of the incident light
* It is the intensity of the transmitted light
The absorbance is directly proportional to the concentration of the analyte and the path length of the light beam through the solution. This relationship is described by Beer’s Law:
A = εbc
where:
* A is the absorbance
* ε is the molar absorptivity, a constant that is specific to the analyte and the wavelength of light used
* b is the path length of the light beam through the solution
* c is the concentration of the analyte
Types of Spectrophotometers
Spectrophotometers come in various types, each with its unique design and applications. Here are some common types:
- UV-Vis Spectrophotometers: These spectrophotometers use ultraviolet (UV) and visible (Vis) light to measure the absorbance of solutions. They are widely used in various applications, including quantitative analysis, kinetic studies, and purity determination.
- Infrared (IR) Spectrophotometers: IR spectrophotometers use infrared radiation to measure the absorbance of molecules. They are particularly useful for identifying and characterizing organic molecules.
- Atomic Absorption Spectrophotometers (AAS): AAS uses the absorption of light by atoms in a gaseous state to determine the concentration of metals in a sample. It is widely used in environmental monitoring, food analysis, and clinical chemistry.
- Flame Photometers: These instruments measure the intensity of light emitted by excited atoms in a flame. They are used to determine the concentration of alkali and alkaline earth metals in samples.
Beer’s Law in Practice
Beer’s Law is not just a theoretical concept; it finds widespread application in various scientific disciplines. It provides a practical method for determining the concentration of a substance in a solution by measuring its absorbance of light.
Performing a Beer’s Law Experiment
A Beer’s Law experiment typically involves a series of steps designed to establish a relationship between absorbance and concentration. This relationship is then used to determine the concentration of an unknown sample.
- Prepare Solutions of Known Concentrations: Start by creating a series of solutions with known concentrations of the analyte. This forms the basis for your calibration curve.
- Measure Absorbance: Use a spectrophotometer to measure the absorbance of each solution at a specific wavelength. The wavelength chosen should correspond to the maximum absorbance of the analyte, ensuring the most sensitive measurements.
- Plot a Calibration Curve: Plot the absorbance values against the corresponding concentrations. This graph is known as the calibration curve. The curve should be linear and pass through the origin, demonstrating the direct relationship between absorbance and concentration as described by Beer’s Law.
- Measure the Absorbance of the Unknown Sample: Use the spectrophotometer to measure the absorbance of the unknown sample at the same wavelength used for the calibration curve.
- Determine the Concentration of the Unknown Sample: Using the calibration curve, find the concentration corresponding to the absorbance of the unknown sample. This can be done by visually interpolating on the graph or using a linear regression equation.
Calibration Curves and Concentration Determination
Calibration curves are essential for determining unknown concentrations using Beer’s Law. They establish the linear relationship between absorbance and concentration for a specific analyte. This relationship is crucial for accurate concentration determination.
- Linearity: The calibration curve should exhibit a linear relationship between absorbance and concentration, ensuring accurate concentration predictions within the range of the curve.
- Range: The calibration curve should cover a suitable range of concentrations relevant to the analyte being analyzed. It should encompass both low and high concentrations to accommodate various sample types.
- Accuracy: The calibration curve should be accurate, with data points closely aligned to the linear relationship. This ensures reliable concentration predictions based on absorbance measurements.
Real-World Applications of Beer’s Law
Beer’s Law has numerous applications in analytical chemistry and related fields, contributing to various scientific and industrial processes.
- Pharmaceutical Analysis: Beer’s Law is widely used in the pharmaceutical industry for quality control and analysis of drug formulations. It helps determine the concentration of active pharmaceutical ingredients in tablets, capsules, and other dosage forms.
- Environmental Monitoring: Beer’s Law is instrumental in environmental monitoring, where it helps determine the concentrations of pollutants in water, soil, and air. This information is vital for assessing environmental health and taking appropriate actions to mitigate pollution.
- Food Chemistry: Beer’s Law is used in food chemistry to analyze the composition of food products, including the concentration of vitamins, minerals, and other nutrients. It helps ensure food quality and safety.
- Clinical Chemistry: Beer’s Law plays a crucial role in clinical chemistry, where it is used to determine the concentrations of various analytes in biological samples, such as blood, urine, and serum. This information is essential for diagnosing and monitoring diseases.
The applications of Beer’s Law are diverse and contribute significantly to various fields, making it a fundamental tool in analytical chemistry and related disciplines.
Ultimate Conclusion
Beer’s Law serves as a powerful tool for quantitative analysis, allowing scientists to accurately determine the concentration of substances in solutions. Its applications span diverse fields, from chemistry and biology to environmental science. Understanding the factors that can influence Beer’s Law measurements, such as non-linearity and interferences, is crucial for ensuring accurate results. By mastering the principles of Beer’s Law and its applications, we gain a deeper understanding of the intricate world of light absorption and its profound implications in various scientific disciplines.
Key Questions Answered
How does Beer’s Law relate to spectrophotometry?
Spectrophotometry is the technique used to measure the absorbance of light by a solution. Beer’s Law is applied in spectrophotometry to determine the concentration of a substance by measuring its absorbance at a specific wavelength.
What are some real-world examples of Beer’s Law applications?
Beer’s Law is used in various applications, such as determining the concentration of glucose in blood samples, analyzing the purity of a pharmaceutical compound, and monitoring environmental pollutants in water samples.
What are some limitations of Beer’s Law?
Beer’s Law has some limitations, such as deviations from linearity at high concentrations, the presence of interferences, and the need for accurate sample preparation.





