
What is Hund’s Law? It’s a fundamental principle in chemistry that governs the arrangement of electrons within atoms. Discovered by German physicist Friedrich Hund in 1927, Hund’s Rule explains how electrons fill orbitals in a way that maximizes the stability of an atom. This rule is essential for understanding atomic structure, chemical bonding, and the magnetic properties of elements.
Imagine electrons as tiny, negatively charged particles orbiting the nucleus of an atom. Each electron occupies a specific energy level and orbital, which can be visualized as a three-dimensional region of space where the electron is most likely to be found. Hund’s Rule dictates that electrons prefer to occupy separate orbitals within a subshell, rather than pairing up in the same orbital, as long as they have the same spin. This rule helps to explain why certain elements are paramagnetic, meaning they are attracted to a magnetic field, while others are diamagnetic, meaning they are repelled by a magnetic field.
Introduction to Hund’s Rule
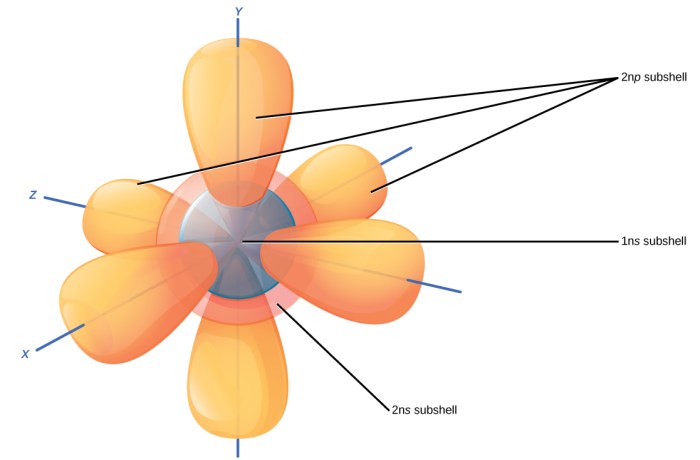
Hund’s Rule, a fundamental principle in chemistry and physics, governs the distribution of electrons within atoms. It describes how electrons fill atomic orbitals in a specific manner, leading to the most stable electronic configuration.
This rule, named after the German physicist Friedrich Hund, who formulated it in 1927, plays a crucial role in understanding the behavior of atoms and molecules.
Historical Context and Discovery
The discovery of Hund’s Rule stemmed from the development of quantum mechanics, which revolutionized our understanding of the atom. Prior to this, scientists knew that electrons occupied specific energy levels within an atom, but they lacked a clear understanding of how these electrons were arranged.
In the 1920s, Hund, building upon the work of other physicists, proposed that electrons would first individually occupy each orbital within a subshell before pairing up in the same orbital. This rule, later known as Hund’s Rule, was based on experimental observations and theoretical calculations.
Significance of Hund’s Rule, What is hund’s law
Hund’s Rule is fundamental to understanding the structure of atoms and molecules. It helps us predict the electronic configuration of atoms, which in turn determines their chemical properties and how they interact with other atoms to form bonds.
Here’s how Hund’s Rule impacts our understanding of atomic structure and chemical bonding:
* Electronic Configuration: It dictates the distribution of electrons in atomic orbitals, explaining the stability of atoms.
* Chemical Bonding: Hund’s Rule influences the formation of chemical bonds, as atoms strive to achieve a stable electron configuration by sharing or transferring electrons.
* Magnetic Properties: The rule also explains the magnetic properties of atoms, as the unpaired electrons in orbitals contribute to the atom’s overall magnetic moment.
Hund’s Rule and Chemical Bonding
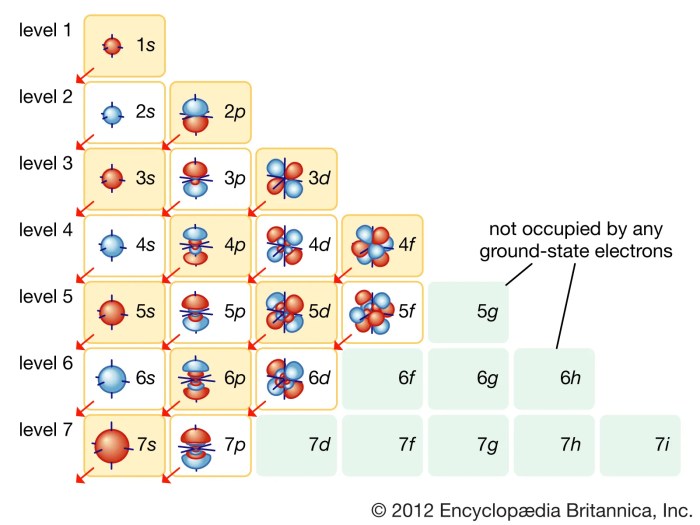
Hund’s Rule, a fundamental principle in atomic physics, plays a crucial role in shaping the formation and stability of chemical bonds. This rule dictates the distribution of electrons within an atom’s orbitals, influencing the way atoms interact with each other and form molecules.
The Influence of Hund’s Rule on Chemical Bond Formation
Hund’s Rule states that electrons will individually occupy each orbital within a subshell before pairing up in any one orbital. This rule ensures that the electrons within an atom are as spatially separated as possible, minimizing electron-electron repulsion. This principle directly impacts the formation of chemical bonds, which involve the sharing or transfer of electrons between atoms.
Atoms with unpaired electrons, as dictated by Hund’s Rule, are more likely to participate in chemical bonding. These unpaired electrons are readily available for sharing or transfer, leading to the formation of stable chemical bonds. Conversely, atoms with all paired electrons in their orbitals are less reactive and less likely to form bonds.
Hund’s Rule and Molecular Stability
The stability of molecules is a direct consequence of the arrangement of electrons within the constituent atoms. Hund’s Rule significantly contributes to molecular stability by influencing the electronic configuration of atoms involved in bonding.
Atoms with unpaired electrons, following Hund’s Rule, can form stronger and more stable bonds. This is because the unpaired electrons can readily participate in bonding, resulting in a more balanced distribution of electrons between the bonding atoms. Conversely, atoms with all paired electrons tend to form weaker bonds due to the increased electron-electron repulsion within the molecule.
Examples of Chemical Bonds Influenced by Hund’s Rule
The influence of Hund’s Rule on chemical bond formation is evident in numerous chemical compounds. Here are some notable examples:
Covalent Bonding
– Oxygen (O2): Oxygen has two unpaired electrons in its outermost p-orbitals. According to Hund’s Rule, these electrons will occupy separate p-orbitals. When two oxygen atoms bond to form oxygen gas (O2), these unpaired electrons form a double covalent bond, leading to a stable molecule.
– Nitrogen (N2): Nitrogen has three unpaired electrons in its outermost p-orbitals. These unpaired electrons participate in triple covalent bond formation, resulting in the exceptionally strong and stable nitrogen molecule (N2).
Ionic Bonding
– Sodium Chloride (NaCl): Sodium (Na) has one unpaired electron in its outermost s-orbital, while chlorine (Cl) has one unpaired electron in its outermost p-orbital. When sodium loses its valence electron to chlorine, forming Na+ and Cl- ions, respectively, they are attracted to each other through electrostatic forces, forming an ionic bond.
These examples highlight how Hund’s Rule directly influences the formation of chemical bonds, contributing to the stability and properties of molecules.
Applications of Hund’s Rule
Hund’s Rule, a fundamental principle in atomic physics and chemistry, has wide-ranging applications in various scientific fields. Its impact extends beyond theoretical understanding, influencing technological advancements and shaping our understanding of the world around us.
Applications of Hund’s Rule in Different Fields
Hund’s Rule finds applications in diverse fields, including chemistry, physics, and materials science. Here’s a table outlining some of its key applications:
| Field | Applications |
|---|---|
| Chemistry |
|
| Physics |
|
| Materials Science |
|
Examples of Hund’s Rule in Chemistry, Physics, and Materials Science
Chemistry
Hund’s Rule plays a crucial role in understanding chemical bonding and reactivity. For example, it helps explain why oxygen is a diatomic molecule (O2) with a double bond, while nitrogen is a diatomic molecule (N2) with a triple bond. This difference in bonding arises from the electronic configurations of oxygen and nitrogen atoms, which are determined by Hund’s Rule.
Physics
In physics, Hund’s Rule is essential for understanding the magnetic properties of materials. For example, ferromagnetism, the phenomenon responsible for the magnetism of iron, is directly related to the alignment of electron spins in accordance with Hund’s Rule. This alignment leads to a net magnetic moment, resulting in the material’s magnetic behavior.
Materials Science
Hund’s Rule has significant implications for the design of new materials. For instance, it is used to develop materials with specific magnetic properties, such as those used in magnetic recording media and data storage devices. Understanding Hund’s Rule allows scientists to predict and control the magnetic behavior of materials, enabling the creation of new technologies.
Impact of Hund’s Rule on Technological Advancements
Hund’s Rule has profoundly impacted technological advancements in various fields. Its applications have led to the development of:
- Magnetic storage devices: Hund’s Rule helps understand the magnetic properties of materials used in hard drives, floppy disks, and magnetic tapes, enabling the development of efficient data storage technologies.
- Catalysts: Hund’s Rule aids in designing catalysts for various chemical reactions, including those used in the production of fuels, pharmaceuticals, and plastics.
- Advanced materials: Hund’s Rule contributes to the development of materials with enhanced strength, durability, and other desired properties, finding applications in aerospace, automotive, and other industries.
Final Conclusion

Hund’s Rule is a powerful tool for understanding the behavior of atoms and molecules. Its implications extend beyond basic chemistry, influencing fields like materials science and spectroscopy. By understanding how electrons fill orbitals, we can predict the chemical properties of elements, design new materials with specific properties, and interpret the complex spectra of atoms and molecules. As we continue to explore the intricacies of atomic structure, Hund’s Rule will undoubtedly play a crucial role in our understanding of the fundamental building blocks of matter.
FAQ Compilation: What Is Hund’s Law
What is the difference between Hund’s Rule and the Aufbau principle?
The Aufbau principle states that electrons fill orbitals in order of increasing energy. Hund’s Rule is a specific rule within the Aufbau principle that focuses on how electrons fill orbitals within a subshell.
What are some examples of elements that exhibit paramagnetism due to Hund’s Rule?
Elements like oxygen (O), nitrogen (N), and copper (Cu) are paramagnetic due to unpaired electrons in their orbitals, as predicted by Hund’s Rule.
How can Hund’s Rule be applied in materials science?
Hund’s Rule helps in understanding the magnetic properties of materials. For example, it helps to explain why certain materials are ferromagnetic, meaning they can be permanently magnetized.
Are there any exceptions to Hund’s Rule?
Yes, there are some exceptions to Hund’s Rule, particularly in cases involving transition metals and lanthanides. These exceptions are due to complex interactions between electrons and the nucleus.





