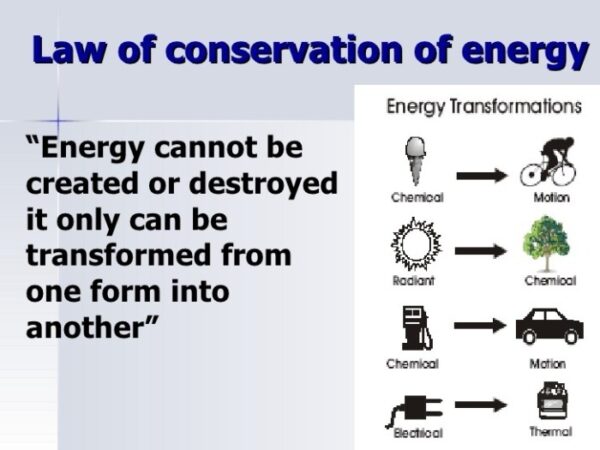
What is law conservation of energy – What is the law of conservation of energy? It’s a fundamental principle in physics that states energy can neither be created nor destroyed, only transformed from one form to another. This concept, dating back to the 18th century, has profound implications for understanding the universe and our place within it. From the simple act of turning on a light switch to the complex processes occurring within our bodies, the law of conservation of energy is constantly at work, shaping our world.
Imagine a child on a swing. As the swing rises, it gains potential energy, stored by its position above the ground. As it falls, this potential energy transforms into kinetic energy, the energy of motion. This simple example illustrates the fundamental principle: energy is not lost but simply changes form. This principle extends to all forms of energy, from the chemical energy stored in food to the nuclear energy powering the sun.
Introduction to the Law of Conservation of Energy
The law of conservation of energy is a fundamental principle in physics that states that energy cannot be created or destroyed, only transformed from one form to another. This means that the total amount of energy in an isolated system remains constant over time, even though it may change forms. This law has far-reaching implications in various fields, from engineering to biology, and is essential for understanding how the universe works.
Historical Context and Key Figures
The concept of energy conservation has roots in ancient philosophical ideas about the nature of the universe. However, the modern understanding of the law of conservation of energy emerged in the 18th and 19th centuries, with contributions from several prominent scientists.
- Julius Robert Mayer (1814-1878): A German physician who, in 1842, published a paper proposing the equivalence of mechanical work and heat. This was a crucial step in recognizing that energy can be transformed between different forms.
- James Prescott Joule (1818-1889): An English physicist who conducted meticulous experiments to demonstrate the relationship between mechanical work and heat. He established the mechanical equivalent of heat, a fundamental constant that quantifies the amount of mechanical work required to produce a given amount of heat.
- Hermann von Helmholtz (1821-1894): A German physiologist and physicist who formulated a comprehensive statement of the law of conservation of energy in 1847. He recognized that energy can exist in various forms, including mechanical, thermal, chemical, and electrical energy, and that it can be converted between these forms.
Real-World Examples
The law of conservation of energy manifests in countless ways in everyday life. Here are some examples:
- A bouncing ball: When a ball is dropped, it possesses potential energy due to its height. As it falls, this potential energy is converted into kinetic energy, the energy of motion. When the ball hits the ground, the kinetic energy is transferred back into potential energy, causing the ball to bounce back up. This process continues until the energy is dissipated through friction and air resistance.
- A hydroelectric dam: A hydroelectric dam utilizes the potential energy of water stored at a higher elevation. As the water flows down through the dam, its potential energy is converted into kinetic energy, which drives turbines to generate electricity.
- A car engine: A car engine converts chemical energy stored in gasoline into mechanical energy to power the vehicle. The combustion process releases heat energy, which is then transformed into mechanical energy to turn the wheels.
Forms of Energy and Their Transformations: What Is Law Conservation Of Energy

Energy is the ability to do work. It exists in various forms, and the Law of Conservation of Energy states that energy cannot be created or destroyed, only transformed from one form to another.
Forms of Energy
Energy manifests in various forms, each with its unique characteristics and applications.
- Potential Energy: This energy is stored due to an object’s position or configuration. For instance, a book held above the ground possesses potential energy due to its height. This energy is released as kinetic energy when the book is dropped.
- Kinetic Energy: This energy is associated with an object’s motion. A moving car, a flowing river, and a spinning top all possess kinetic energy. The faster the object moves, the greater its kinetic energy.
- Thermal Energy: This energy is related to the internal motion of atoms and molecules within a substance. The higher the temperature of a substance, the greater its thermal energy. Heat transfer, a form of thermal energy transfer, occurs when there is a temperature difference between two objects.
- Chemical Energy: This energy is stored in the bonds between atoms and molecules. The breaking and forming of chemical bonds release or absorb chemical energy. For example, burning fuel releases chemical energy in the form of heat and light.
- Nuclear Energy: This energy is stored in the nucleus of an atom. Nuclear reactions, such as fission and fusion, release tremendous amounts of energy. Nuclear power plants harness this energy to generate electricity.
Energy Transformations
Energy transformations occur when energy changes from one form to another. This process is governed by the Law of Conservation of Energy, meaning the total amount of energy remains constant.
- Burning Fuel: Burning fuel, like wood or gasoline, converts chemical energy stored in the fuel’s bonds into thermal energy (heat) and light energy. This process is used to power vehicles and generate electricity.
- Hydroelectric Power Generation: Hydroelectric power plants convert the potential energy of water stored at a higher elevation into kinetic energy as it flows downhill. This kinetic energy then drives turbines, which in turn generate electricity.
- Solar Power Generation: Solar panels convert light energy from the sun into electrical energy. This process involves the photoelectric effect, where photons from sunlight strike a material, causing electrons to be emitted and generating an electric current.
Energy Efficiency
Energy efficiency refers to the ratio of useful energy output to total energy input. In energy transformations, some energy is always lost as heat or other forms of unusable energy. Energy efficiency aims to minimize these losses, maximizing the useful output of energy.
Energy efficiency is crucial for sustainability, reducing energy consumption and minimizing environmental impact.
Applications of the Law of Conservation of Energy

The Law of Conservation of Energy, a fundamental principle in physics, has far-reaching implications and applications across various fields, influencing our understanding of the universe and shaping technological advancements. It serves as a cornerstone for numerous scientific and engineering disciplines, providing a framework for analyzing and predicting energy transformations in diverse systems.
Applications in Different Fields
The law of conservation of energy finds practical applications in various fields, contributing to advancements in technology, scientific research, and our understanding of natural phenomena. Here’s a glimpse of its diverse applications:
| Field | Applications | Examples |
|---|---|---|
| Engineering |
|
|
| Physics |
|
|
| Biology |
|
|
Role in Addressing Global Energy Challenges
The law of conservation of energy plays a pivotal role in addressing global energy challenges by guiding the development of sustainable energy solutions. It emphasizes the importance of efficient energy utilization and encourages the exploration of renewable energy sources.
“The total energy of an isolated system remains constant; it is said to be conserved over time.”
The law of conservation of energy serves as a guiding principle for developing energy-efficient technologies, reducing energy waste, and promoting the use of renewable energy sources. By understanding the principles of energy conservation, we can make informed decisions about energy consumption, production, and distribution, contributing to a more sustainable future.
Implications and Misconceptions
The Law of Conservation of Energy, a fundamental principle in physics, has profound implications for understanding and predicting the behavior of physical systems. It is also subject to various misconceptions that can lead to misunderstandings. This section explores these implications and misconceptions, providing a clearer understanding of the law’s scope and limitations.
Comparison with Other Scientific Principles
The Law of Conservation of Energy is closely related to other fundamental scientific principles, such as the laws of motion and thermodynamics. These principles share similarities and differences that are important to understand.
- Newton’s Laws of Motion: These laws describe the relationship between force, mass, and acceleration. While the Law of Conservation of Energy focuses on the total amount of energy in a system, Newton’s laws describe how energy is transferred and transformed through forces. For instance, the Law of Conservation of Energy states that the total energy of a system remains constant, while Newton’s Second Law explains how force causes a change in an object’s momentum, which is directly related to its kinetic energy.
- Thermodynamics: Thermodynamics deals with heat, work, and energy transfer in physical systems. The First Law of Thermodynamics is essentially a restatement of the Law of Conservation of Energy, emphasizing the equivalence of heat and work. The Second Law of Thermodynamics, however, introduces the concept of entropy, which states that the total entropy of an isolated system always increases over time. This implies that while energy is conserved, its availability for useful work decreases during energy transformations.
Common Misconceptions
Despite its fundamental nature, the Law of Conservation of Energy is often misunderstood. Here are some common misconceptions and their clarifications:
- Energy can be created or destroyed: This is a common misconception. The Law of Conservation of Energy explicitly states that energy cannot be created or destroyed, only transformed from one form to another.
- Energy is always conserved in all processes: While the Law of Conservation of Energy holds true for most everyday processes, it has limitations in certain scenarios, such as nuclear reactions. In nuclear reactions, a small amount of mass is converted into energy, seemingly violating the law. However, this is explained by Einstein’s famous equation E=mc², which shows that mass and energy are equivalent and can be interconverted.
- Energy efficiency means that energy is not conserved: Energy efficiency does not violate the Law of Conservation of Energy. It simply means that less energy is wasted during a process. For example, a more efficient car engine converts more of the fuel’s chemical energy into mechanical energy, reducing the amount of energy lost as heat. The total energy, however, remains conserved.
Limitations of the Law of Conservation of Energy, What is law conservation of energy
While the Law of Conservation of Energy is a fundamental principle, it has certain limitations in specific scenarios.
- Nuclear Reactions: As mentioned earlier, nuclear reactions involve the conversion of a small amount of mass into energy. This process, governed by Einstein’s E=mc², seemingly violates the Law of Conservation of Energy. However, the principle still holds true if we consider the mass-energy equivalence.
- Open Systems: The Law of Conservation of Energy applies to closed systems, where no energy is exchanged with the surroundings. In open systems, energy can be exchanged with the environment, making it difficult to track the total energy of the system. For example, in a chemical reaction, energy is released or absorbed from the surroundings.
- Quantum Mechanics: At the quantum level, the Law of Conservation of Energy is not strictly obeyed. In certain quantum processes, such as the decay of radioactive isotopes, energy can be temporarily borrowed or lent, leading to seemingly non-conservative behavior. However, this behavior is still explained by the principles of quantum mechanics and does not invalidate the fundamental principle of energy conservation.
Summary
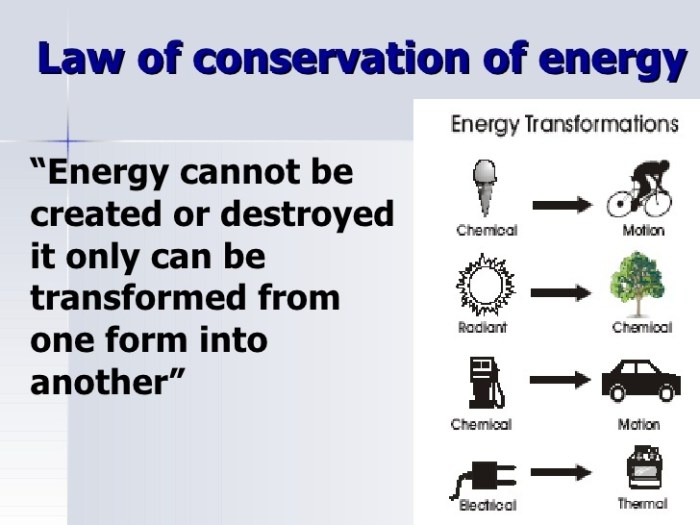
The law of conservation of energy is a cornerstone of modern science, guiding our understanding of everything from the smallest particles to the vastness of space. While it has its limitations, its applications are vast, influencing fields as diverse as engineering, physics, and biology. As we continue to explore the universe and develop new technologies, the law of conservation of energy will remain a guiding principle, reminding us of the fundamental interconnectedness of all things.
FAQ Resource
Can energy be destroyed?
No, according to the law of conservation of energy, energy cannot be destroyed, only transformed from one form to another. This means that the total amount of energy in a closed system remains constant.
What are some real-world examples of energy transformations?
Examples include burning fuel, which converts chemical energy into heat and light, or hydroelectric power generation, which converts the potential energy of water stored at a higher elevation into kinetic energy as it flows and then into electrical energy.
Does the law of conservation of energy apply to all forms of energy?
Yes, the law applies to all forms of energy, including potential, kinetic, thermal, chemical, and nuclear energy.