
What is the law of definite proportions? This fundamental law in chemistry, discovered in the late 18th century, reveals a fascinating truth about the composition of chemical compounds. It states that a given chemical compound always contains the same elements in the same proportion by mass, regardless of its source or method of preparation. This principle, established by scientists like Joseph Proust, laid the groundwork for our understanding of chemical reactions and the nature of matter itself.
Imagine you have two samples of water, one collected from a pristine mountain spring and the other from a polluted city river. While their origins may differ, the law of definite proportions tells us that both samples will always contain the same elements – hydrogen and oxygen – in the same precise ratio by mass. This unwavering consistency is a cornerstone of chemistry, allowing us to predict and understand the behavior of chemical substances.
Introduction to the Law of Definite Proportions

The Law of Definite Proportions is a fundamental principle in chemistry that governs the composition of chemical compounds. It states that a given chemical compound always contains the same elements in the same proportions by mass, regardless of its source or method of preparation. This law played a crucial role in the development of modern chemistry, paving the way for our understanding of chemical reactions and the nature of matter.
The Historical Context of the Law’s Discovery
The Law of Definite Proportions emerged from a series of meticulous experiments conducted by scientists in the late 18th and early 19th centuries. The discovery of this law can be traced back to the work of several key figures:
- Joseph Proust (1754-1826), a French chemist, is widely credited with the first clear statement of the law. Through his experiments on various compounds, including copper carbonate, he observed that the proportions of the elements in the compound remained constant, regardless of the source or method of preparation. He published his findings in 1799, laying the foundation for the Law of Definite Proportions.
- John Dalton (1766-1844), an English chemist and physicist, further solidified the law with his atomic theory. Dalton proposed that elements are composed of indivisible particles called atoms, and that chemical compounds are formed by the combination of atoms in fixed ratios. This theory provided a theoretical framework for the Law of Definite Proportions, explaining why the proportions of elements in a compound remain constant.
Definition of the Law of Definite Proportions
The Law of Definite Proportions can be defined as follows:
A given chemical compound always contains the same elements in the same proportions by mass, regardless of its source or method of preparation.
This means that no matter where you obtain a particular compound, whether it’s from a natural source, a laboratory synthesis, or any other method, the ratio of the masses of its constituent elements will always be the same. For example, water (H2O) will always contain 11.19% hydrogen and 88.81% oxygen by mass, regardless of its source or method of preparation.
The Law’s Core Principle

The Law of Definite Proportions, also known as Proust’s Law, is a fundamental principle in chemistry that states that a chemical compound always contains the same elements in the same proportion by mass, regardless of its source or method of preparation.
This principle is based on the fact that chemical compounds are formed by the combination of atoms in fixed ratios. For example, water (H2O) always contains two hydrogen atoms for every one oxygen atom, regardless of whether it is obtained from a river, a well, or a laboratory. This means that the mass ratio of hydrogen to oxygen in water will always be the same.
Examples of Chemical Compounds
The Law of Definite Proportions can be illustrated by several examples of chemical compounds:
- Water (H2O): The mass ratio of hydrogen to oxygen in water is always 1:8, regardless of its source. This means that for every 1 gram of hydrogen, there will be 8 grams of oxygen in water.
- Carbon Dioxide (CO2): The mass ratio of carbon to oxygen in carbon dioxide is always 3:8. This means that for every 3 grams of carbon, there will be 8 grams of oxygen in carbon dioxide.
- Sodium Chloride (NaCl): The mass ratio of sodium to chlorine in sodium chloride is always 23:35.5. This means that for every 23 grams of sodium, there will be 35.5 grams of chlorine in sodium chloride.
Constant Mass Ratios of Elements
The following table shows the constant mass ratios of elements in various compounds:
| Compound | Elements | Mass Ratio |
|---|---|---|
| Water (H2O) | Hydrogen (H): Oxygen (O) | 1:8 |
| Carbon Dioxide (CO2) | Carbon (C): Oxygen (O) | 3:8 |
| Sodium Chloride (NaCl) | Sodium (Na): Chlorine (Cl) | 23:35.5 |
| Glucose (C6H12O6) | Carbon (C): Hydrogen (H): Oxygen (O) | 6:1:8 |
The Law of Definite Proportions is a cornerstone of chemistry, providing a basis for understanding the composition and properties of chemical compounds. It also has important implications for chemical analysis, synthesis, and industrial processes.
Applications of the Law
The law of definite proportions, also known as Proust’s law, has profound implications for various fields, particularly in chemistry. Its applications extend beyond theoretical understanding, impacting practical aspects of chemical analysis, purity assessment, and the comprehension of chemical reactions.
Chemical Analysis
The law of definite proportions is a cornerstone of chemical analysis, serving as a fundamental principle in determining the composition of substances. By understanding the fixed ratio of elements in a compound, chemists can accurately quantify the amounts of each element present in a sample. This principle is applied in various analytical techniques, such as:
- Gravimetric analysis: This method involves separating and weighing the components of a compound. The law of definite proportions ensures that the mass of each element in the compound is directly proportional to its fixed ratio, enabling precise determination of the compound’s composition.
- Titration: Titration is a technique used to determine the concentration of a solution by reacting it with a solution of known concentration. The law of definite proportions plays a crucial role in calculating the amount of the unknown substance based on the known stoichiometry of the reaction.
- Elemental analysis: Elemental analysis involves determining the elemental composition of a substance. The law of definite proportions ensures that the percentage of each element in a compound remains constant, allowing for accurate determination of its elemental composition.
Determining the Purity of Substances
The law of definite proportions is instrumental in assessing the purity of substances. If the composition of a sample deviates from the fixed ratio expected for a pure compound, it indicates the presence of impurities. This principle is applied in various industries, including:
- Pharmaceutical industry: Ensuring the purity of drugs is paramount for safety and efficacy. The law of definite proportions is used to verify the composition of pharmaceutical products, ensuring they meet regulatory standards.
- Food industry: The purity of food ingredients is essential for quality and safety. The law of definite proportions is used to analyze the composition of food products, ensuring they meet regulatory standards and maintain consistent quality.
- Industrial chemistry: The purity of raw materials and products is crucial in industrial processes. The law of definite proportions is used to control the quality of industrial chemicals, ensuring consistent performance and minimizing variability.
Understanding Chemical Reactions
The law of definite proportions provides a framework for understanding the stoichiometry of chemical reactions. It explains why chemical reactions occur in specific proportions and why the products formed always have a fixed composition. This principle is crucial for:
- Balancing chemical equations: The law of definite proportions ensures that the number of atoms of each element on the reactants’ side of a chemical equation must equal the number of atoms of that element on the products’ side. This principle is essential for balancing chemical equations, ensuring that mass is conserved during chemical reactions.
- Predicting the yield of a reaction: By knowing the fixed ratio of reactants and products in a chemical reaction, chemists can predict the theoretical yield of a product based on the amount of reactants used. This principle is essential for optimizing chemical processes and maximizing product yield.
- Understanding the mechanism of a reaction: The law of definite proportions provides insights into the mechanism of a chemical reaction by highlighting the fixed ratio of reactants and products involved. This understanding is crucial for designing and optimizing chemical processes.
Illustrative Examples: What Is The Law Of Definite Proportions
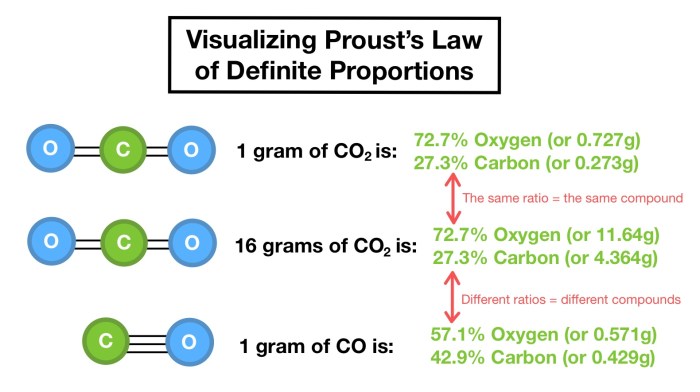
The Law of Definite Proportions is best understood through practical examples. These examples demonstrate the constant mass ratios in chemical compounds and how this principle can be used to determine the composition of a compound or identify an unknown substance.
Visual Representation of Constant Mass Ratios
To illustrate the constant mass ratios in a chemical compound, consider the example of water (H2O). Water is always composed of two hydrogen atoms and one oxygen atom, regardless of its source. This constant ratio of elements leads to a consistent mass ratio.
Here’s a table representing the mass ratio of hydrogen and oxygen in water:
| Element | Mass (g) | Ratio |
|---|---|---|
| Hydrogen (H) | 2 | 1:8 |
| Oxygen (O) | 16 |
This table demonstrates that for every 2 grams of hydrogen, there are 16 grams of oxygen in water. This ratio remains constant, even if we have different quantities of water. For instance, if we have 10 grams of water, it will still have a 1:8 mass ratio of hydrogen to oxygen.
Determining the Composition of a Compound
Let’s say we have a sample of an unknown compound, and we know it contains only carbon (C) and oxygen (O). We can use the Law of Definite Proportions to determine the composition of this compound.
First, we analyze the sample and find that it contains 3 grams of carbon and 8 grams of oxygen. To determine the ratio of carbon to oxygen, we divide the mass of each element by its atomic mass:
– Carbon: 3 g / 12 g/mol = 0.25 mol
– Oxygen: 8 g / 16 g/mol = 0.5 mol
The ratio of carbon to oxygen is 0.25:0.5, which simplifies to 1:2. This indicates that the compound contains one carbon atom for every two oxygen atoms. The formula for the compound is therefore CO2, which is carbon dioxide.
Identifying an Unknown Substance
Imagine you have two samples of white powder. One is known to be table salt (NaCl), and the other is an unknown substance. You can use the Law of Definite Proportions to help you identify the unknown substance.
You analyze both samples and find that the known salt sample contains 39.34% sodium (Na) and 60.66% chlorine (Cl) by mass. Now, you analyze the unknown sample and find that it contains 40% sodium (Na) and 60% chlorine (Cl) by mass.
Since the unknown sample has a very similar mass ratio of sodium to chlorine as the known salt sample, you can confidently conclude that the unknown sample is also table salt (NaCl). The Law of Definite Proportions allows you to identify the unknown substance by comparing its mass ratios to known compounds.
Relationship to Other Laws
The law of definite proportions is closely related to another fundamental law in chemistry, the law of multiple proportions. These laws, developed in the late 18th and early 19th centuries, laid the groundwork for the modern atomic theory and revolutionized our understanding of chemical reactions.
Understanding the relationship between these laws helps us appreciate how they contribute to the fundamental principles of chemistry.
Comparison and Contrast
The law of definite proportions states that a chemical compound always contains the same elements in the same proportions by mass, regardless of the source of the compound. In contrast, the law of multiple proportions states that when two elements combine to form more than one compound, the masses of one element that combine with a fixed mass of the other element are in a simple ratio.
- The law of definite proportions focuses on the fixed composition of a specific compound.
- The law of multiple proportions considers the different combinations of elements forming multiple compounds.
Contribution to the Foundation of Modern Chemistry
These laws provided crucial evidence for the existence of atoms and molecules, laying the foundation for modern chemistry. They demonstrated that matter is not infinitely divisible but consists of fundamental units called atoms.
- The law of definite proportions supported the idea that atoms of different elements combine in fixed ratios to form compounds.
- The law of multiple proportions further strengthened this concept by showing that when elements combine in different ratios, they form different compounds.
Historical Significance, What is the law of definite proportions
The development of these laws was a significant milestone in the history of chemistry. They played a crucial role in the development of Dalton’s atomic theory, which revolutionized our understanding of the nature of matter.
“John Dalton’s atomic theory, proposed in 1803, stated that all matter is composed of atoms, which are indivisible and indestructible particles. The law of definite proportions and the law of multiple proportions provided experimental evidence for Dalton’s theory.”
Last Recap
The law of definite proportions, a testament to the orderly nature of the universe, serves as a cornerstone of chemistry. It provides a framework for understanding the composition of chemical compounds, enabling us to analyze substances, determine their purity, and predict the outcomes of chemical reactions. This foundational law, along with other fundamental principles, continues to shape our understanding of the world around us.
FAQ Overview
How does the law of definite proportions relate to the law of multiple proportions?
The law of definite proportions focuses on the fixed composition of a specific compound, while the law of multiple proportions deals with the different mass ratios of elements when they form multiple compounds. Both laws contribute to the understanding of chemical reactions and the composition of matter.
Can the law of definite proportions be used to identify unknown substances?
Yes, by analyzing the mass ratios of elements in an unknown substance and comparing them to known values for various compounds, the law of definite proportions can help identify the unknown substance.
What are some real-world applications of the law of definite proportions?
The law of definite proportions is crucial in various fields, including pharmaceutical production, chemical analysis, and environmental monitoring. It helps ensure the consistency and purity of substances used in these areas.




