
What is the periodic law sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail and brimming with originality from the outset. The periodic law, a fundamental principle in chemistry, governs the arrangement and properties of elements, revealing a fascinating order within the seemingly chaotic world of atoms.
Imagine a grand library where every book is meticulously organized based on its content. The periodic table, a visual representation of the periodic law, acts as this library for elements, showcasing their unique characteristics and relationships. This remarkable system, developed over centuries by brilliant minds, allows us to understand the building blocks of our universe and predict their behavior in various chemical reactions.
The Foundation of the Periodic Law
The periodic law is a fundamental principle in chemistry that governs the arrangement and properties of elements. It states that the properties of elements are periodic functions of their atomic numbers. This means that elements with similar properties appear at regular intervals in the periodic table. The development of this law was a gradual process, driven by the observations and insights of numerous scientists over several decades.
Early Attempts at Classification
The periodic law did not emerge overnight. It was the culmination of years of scientific inquiry and the work of many scientists. Early attempts to organize elements were based on their observed properties.
- Johann Wolfgang Döbereiner (1817): Döbereiner noticed that certain elements, like chlorine, bromine, and iodine, shared similar chemical properties. He grouped these elements into triads, where the atomic weight of the middle element was approximately the average of the other two. This observation laid the foundation for the idea that elements could be grouped based on their properties.
- John Newlands (1864): Newlands proposed the “Law of Octaves.” He arranged elements in order of increasing atomic weight and observed that every eighth element had similar properties. He likened this pattern to the musical scale, where every eighth note is an octave higher. While Newlands’ Law of Octaves was an important step, it was not universally accepted, as it did not work well for all elements.
- Lothar Meyer (1869): Meyer, independently of Mendeleev, also developed a periodic table based on the atomic weights of elements. He plotted various properties of elements against their atomic weights and observed periodic trends. Meyer’s work provided further evidence for the periodic nature of elements.
Mendeleev’s Contribution
Dmitri Mendeleev, a Russian chemist, played a pivotal role in establishing the periodic law. He is credited with developing the first widely accepted periodic table.
- Mendeleev’s Periodic Table (1869): Mendeleev arranged elements in order of increasing atomic weight, but he also took into account their chemical properties. He grouped elements with similar properties together, forming columns in his table. Importantly, Mendeleev recognized that there were gaps in his table and he boldly predicted the existence of undiscovered elements. He even predicted the properties of these missing elements, which were later confirmed by experimental findings.
- Prediction of New Elements: Mendeleev’s predictions were remarkably accurate. For example, he predicted the existence of an element called eka-aluminum (later named gallium) with an atomic weight of 68, a density of 5.9 g/cm3, and a melting point of 30°C. When gallium was discovered in 1875, its properties closely matched Mendeleev’s predictions. This success solidified the validity of Mendeleev’s periodic law and his periodic table.
The Periodic Table
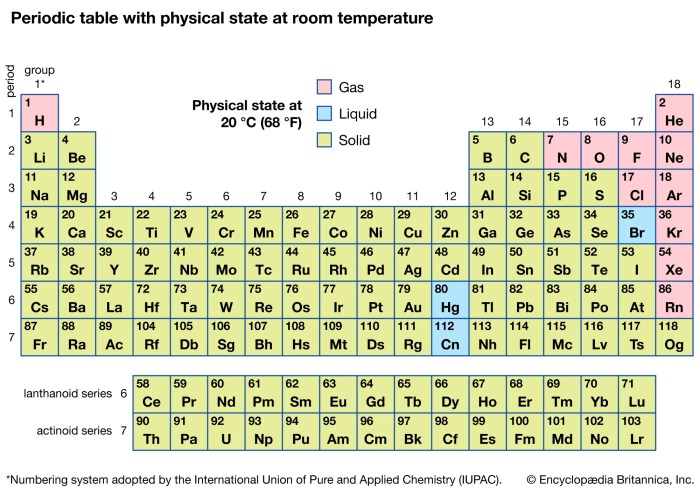
The periodic table is a visual representation of the periodic law, which states that the properties of elements are periodic functions of their atomic numbers. It organizes all known elements based on their atomic number, electron configuration, and recurring chemical properties. This arrangement provides a systematic framework for understanding the relationships between elements and predicting their behavior.
Structure of the Periodic Table
The periodic table is arranged in a grid with rows and columns. Each row represents a period, and each column represents a group.
- Periods: The rows of the periodic table are called periods. Elements within a period have the same number of electron shells. Moving across a period, the atomic number increases, and the number of electrons in the outermost shell, also known as the valence shell, increases. This leads to changes in their chemical properties. For example, elements in the second period (Li, Be, B, C, N, O, F, Ne) all have two electron shells.
- Groups: The columns of the periodic table are called groups. Elements within a group share similar chemical properties due to having the same number of valence electrons. For example, elements in Group 1 (alkali metals) all have one valence electron, which contributes to their high reactivity.
Arrangement of Elements
The periodic table is arranged based on increasing atomic number, which is the number of protons in an atom’s nucleus. The atomic number dictates the element’s identity. The arrangement also reflects the elements’ electron configurations, which describe the distribution of electrons in energy levels. Elements with similar electron configurations tend to exhibit similar chemical properties.
Main Groups and Their Properties
The periodic table is divided into main groups and transition metals. Main groups are further divided into representative elements. Here’s a table highlighting the main groups and their characteristic properties:
| Group | Name | Characteristic Properties | Example |
|---|---|---|---|
| 1 | Alkali Metals | Highly reactive metals, soft, silvery-white, good conductors of heat and electricity | Lithium (Li), Sodium (Na), Potassium (K) |
| 2 | Alkaline Earth Metals | Reactive metals, harder than alkali metals, good conductors of heat and electricity | Beryllium (Be), Magnesium (Mg), Calcium (Ca) |
| 13 | Boron Group | Metalloids, semiconductors, diverse properties | Boron (B), Aluminum (Al), Gallium (Ga) |
| 14 | Carbon Group | Nonmetals, metalloids, and metals, diverse properties | Carbon (C), Silicon (Si), Germanium (Ge) |
| 15 | Nitrogen Group | Nonmetals, metalloids, and metals, diverse properties | Nitrogen (N), Phosphorus (P), Arsenic (As) |
| 16 | Oxygen Group (Chalcogens) | Nonmetals, metalloids, and metals, diverse properties | Oxygen (O), Sulfur (S), Selenium (Se) |
| 17 | Halogens | Highly reactive nonmetals, colorful, form salts with metals | Fluorine (F), Chlorine (Cl), Bromine (Br) |
| 18 | Noble Gases | Colorless, odorless, unreactive gases, found in trace amounts in the atmosphere | Helium (He), Neon (Ne), Argon (Ar) |
Trends in the Periodic Table
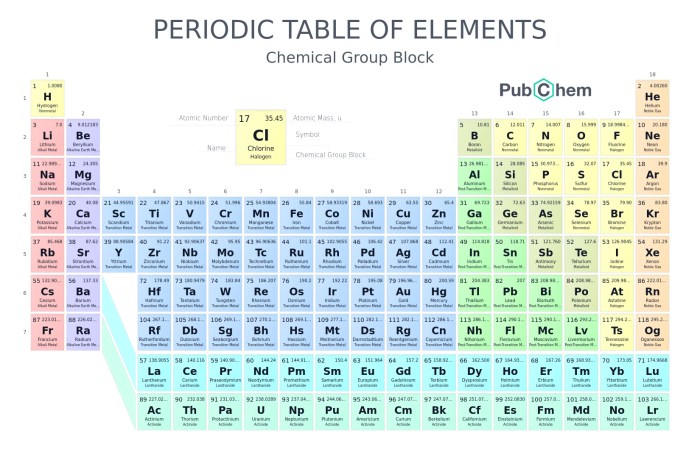
The periodic table is a powerful tool for understanding the properties of elements. It reveals recurring patterns in the behavior of elements, known as periodic trends. These trends are directly related to the arrangement of electrons in atoms, particularly the valence electrons, which are the outermost electrons involved in chemical bonding.
Atomic Radius, What is the periodic law
Atomic radius refers to the distance between the nucleus of an atom and its outermost electron shell. The atomic radius of an element influences its ability to form bonds with other atoms.
The atomic radius generally decreases across a period from left to right. This is because the number of protons in the nucleus increases, leading to a stronger attraction between the nucleus and the electrons. This stronger attraction pulls the electrons closer to the nucleus, decreasing the atomic radius.
The atomic radius generally increases down a group. This is because the number of electron shells increases, meaning the outermost electrons are further from the nucleus. The additional electron shells shield the outermost electrons from the nucleus, reducing the attraction between them.
Ionization Energy
Ionization energy is the minimum amount of energy required to remove an electron from a gaseous atom in its ground state. This energy is a measure of the atom’s ability to hold onto its electrons.
Ionization energy generally increases across a period from left to right. This is because the increasing nuclear charge attracts the electrons more strongly, making it more difficult to remove an electron.
Ionization energy generally decreases down a group. This is because the outermost electrons are further from the nucleus and are shielded by inner electrons, making them easier to remove.
Electronegativity
Electronegativity is a measure of an atom’s ability to attract electrons towards itself when it forms a chemical bond. It is a crucial factor in determining the type of bond that will form between two atoms.
Electronegativity generally increases across a period from left to right. This is because the increasing nuclear charge pulls the electrons more strongly towards the nucleus, making the atom more electronegative.
Electronegativity generally decreases down a group. This is because the outermost electrons are further from the nucleus and are shielded by inner electrons, making them less attracted to the nucleus and reducing the atom’s electronegativity.
Electron Affinity
Electron affinity is the change in energy that occurs when an electron is added to a neutral gaseous atom to form a negative ion. It is a measure of the atom’s tendency to gain an electron.
Electron affinity generally increases across a period from left to right. This is because the increasing nuclear charge attracts the added electron more strongly, resulting in a more negative electron affinity.
Electron affinity generally decreases down a group. This is because the outermost electrons are further from the nucleus and are shielded by inner electrons, reducing the attraction for the added electron.
Table Illustrating Periodic Trends
| Trend | Across a Period (Left to Right) | Down a Group |
|---|---|---|
| Atomic Radius | Decreases | Increases |
| Ionization Energy | Increases | Decreases |
| Electronegativity | Increases | Decreases |
The Periodic Law and Chemical Properties
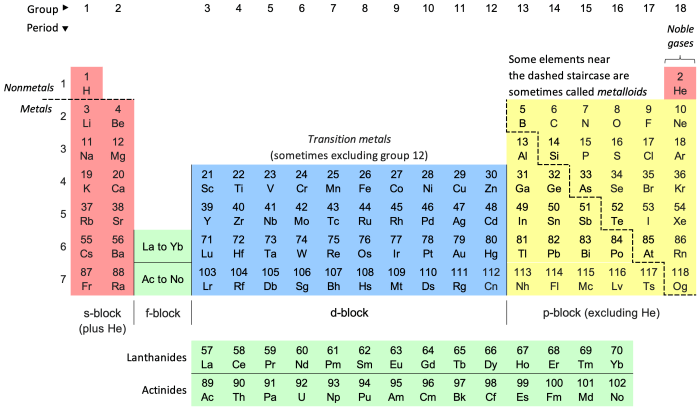
The periodic law, with its foundation in the arrangement of elements based on their atomic numbers and recurring properties, provides a powerful framework for understanding and predicting the chemical behavior of elements. This section explores the intricate relationship between the periodic law and chemical properties, revealing how the periodic table serves as a roadmap for understanding the chemical world.
The Periodic Law and Chemical Bonding
The periodic law plays a crucial role in understanding the formation of chemical bonds, the forces that hold atoms together in molecules and compounds. The arrangement of electrons in an atom’s outermost shell, known as the valence shell, determines its bonding behavior. The periodic law helps us understand the distribution of valence electrons and how this distribution influences the types of bonds that an element can form.
For instance, elements in Group 1 (alkali metals) have one valence electron, making them highly reactive and prone to losing this electron to form ionic bonds with nonmetals. In contrast, elements in Group 17 (halogens) have seven valence electrons, making them highly electronegative and eager to gain an electron to complete their octet. This tendency leads them to form ionic bonds with metals or covalent bonds with other nonmetals.
Reactivity of Elements
The periodic law also explains the trends in reactivity observed among elements. The reactivity of an element is its tendency to participate in chemical reactions.
Reactivity in Groups
Elements within the same group (vertical column) of the periodic table share similar chemical properties because they have the same number of valence electrons. This similarity in valence electron configuration leads to predictable patterns in their reactivity.
- Elements in Group 1 (alkali metals) are highly reactive due to their single valence electron, readily lost to form positive ions. Their reactivity increases as you move down the group due to the increasing atomic size and decreasing ionization energy.
- Elements in Group 17 (halogens) are also highly reactive due to their seven valence electrons, readily gained to form negative ions. Their reactivity decreases as you move down the group due to the increasing atomic size and decreasing electronegativity.
Reactivity in Periods
Elements within the same period (horizontal row) of the periodic table have different numbers of valence electrons, leading to varying reactivity patterns.
- As you move across a period from left to right, the number of valence electrons increases, leading to a general increase in electronegativity and a decrease in metallic character. This trend explains why elements on the left side of the periodic table are typically metals, while those on the right side are nonmetals.
- The reactivity of metals generally decreases as you move across a period, while the reactivity of nonmetals generally increases. This trend is due to the increasing nuclear charge and decreasing atomic size, making it harder for metals to lose electrons and easier for nonmetals to gain electrons.
Applications of the Periodic Law
The periodic law, with its organization of elements based on their properties, serves as a powerful tool in numerous scientific fields. Its applications extend beyond basic understanding of chemical behavior, influencing the development of new materials, technologies, and industrial processes.
Applications in Chemistry
The periodic law is fundamental to the study of chemistry, providing a framework for understanding chemical reactions, predicting the behavior of elements, and designing new compounds.
- Predicting Chemical Reactions: The periodic table helps predict the type of chemical reactions elements will undergo based on their group and period. For example, elements in the same group tend to exhibit similar reactivity patterns, making it possible to anticipate the products of reactions involving them.
- Designing New Compounds: The periodic law guides the synthesis of new compounds with specific properties. By understanding the relationship between an element’s position on the periodic table and its properties, chemists can strategically choose elements to create materials with desired characteristics, such as high conductivity, specific optical properties, or enhanced catalytic activity.
- Understanding Chemical Bonding: The periodic law provides insights into the formation of chemical bonds between atoms. The electronegativity of elements, which influences the type of bond formed, is directly related to their position on the periodic table.
Applications in Physics
The periodic law plays a crucial role in understanding the behavior of atoms and their interactions with electromagnetic radiation.
- Atomic Spectra: The periodic table helps explain the patterns observed in atomic spectra. The wavelengths of light emitted or absorbed by an atom are related to the energy levels of its electrons, which are influenced by the element’s position on the periodic table.
- Nuclear Physics: The periodic law is relevant to nuclear physics, particularly in understanding nuclear reactions and the stability of isotopes. The number of protons and neutrons in an atom’s nucleus, which determines its atomic number and isotopic identity, are directly related to the element’s position on the periodic table.
Applications in Materials Science
The periodic law is indispensable in materials science, enabling the design and development of materials with specific properties for various applications.
- Semiconductors: The periodic law is critical in the development of semiconductors, materials used in electronic devices. The properties of semiconductors, such as their conductivity, are influenced by the electronic configuration of the elements they contain.
- Superconductors: The periodic law guides the search for new superconducting materials, which have zero electrical resistance at low temperatures. The discovery of high-temperature superconductors, such as those based on copper oxides, has been aided by understanding the electronic structure of elements in the periodic table.
- Ceramics: The periodic law is essential in the development of ceramics, materials known for their strength, heat resistance, and electrical insulation properties. The composition and properties of ceramics are influenced by the chemical bonding between the elements they contain, which can be predicted using the periodic law.
Applications in Industry
The periodic law has wide-ranging applications in various industries, influencing the development of new technologies and processes.
| Industry | Applications | Examples |
|---|---|---|
| Electronics | Development of semiconductors, transistors, and integrated circuits | Silicon (Si), germanium (Ge), gallium arsenide (GaAs) |
| Energy | Development of batteries, fuel cells, and solar cells | Lithium (Li), nickel (Ni), cobalt (Co) |
| Pharmaceuticals | Synthesis of new drugs and drug delivery systems | Platinum (Pt), gold (Au), palladium (Pd) |
| Agriculture | Development of fertilizers and pesticides | Nitrogen (N), phosphorus (P), potassium (K) |
| Construction | Production of cement, concrete, and steel | Calcium (Ca), iron (Fe), aluminum (Al) |
Ending Remarks: What Is The Periodic Law
From predicting the reactivity of elements to designing new materials, the periodic law serves as a powerful tool for scientific advancement. As we delve deeper into the intricacies of the periodic table, we unlock a universe of knowledge, unveiling the secrets of the elements and their interconnectedness. It is a testament to the elegance and beauty of nature, demonstrating the underlying order that governs our world.
Q&A
What are some common applications of the periodic law?
The periodic law has numerous applications across various fields, including:
- Material science: Predicting the properties of new materials, such as semiconductors and superconductors.
- Industrial processes: Optimizing chemical reactions and developing new catalysts.
- Environmental science: Understanding the behavior of pollutants and developing strategies for remediation.
- Medicine: Developing new drugs and therapies.
How does the periodic law help us understand chemical reactions?
The periodic law provides insights into the reactivity of elements by revealing their electron configurations. Elements in the same group tend to have similar chemical properties due to their similar outer electron configurations. This understanding helps predict how elements will interact and form chemical bonds.




